深圳市安科瑞仪器有限公司成立于2013年,是一家集研发、生产、销售、服务为一体的高科技企业。公司产品主要涵盖家用医疗器械、家用保健器械、穿戴式医疗器械三大领域几十种型号,已进入持续快速成长健康通道。公司以为人类带来健康生活为导向,以研发创新为基石,采用领先的技术开发出创新的医疗健康产品。
Shenzhen Acurio Instrument Co., LTD
Acurio, founded in 2013, is one of the leading manufacturers which specialize in medical equipment. Our products include Fingertip Pulse Oximeters, Compressor Nebulizers, Fetal Dopplers, Blood Pressure Monitors and Infrared Thermometers.
We have 150+ intelligent, self-driven, and innovative people working in the company, the office of R&D and sales is located in the high-tech city of Shenzhen, and our factory is located in the beautiful seaside city of Xiamen.
Acurio takes safety, accuracy, and reliability as essential considerations in development and manufacturing. We design according to CE and FDA standards, and manufacture under the requirements of the ISO 13485 system.
Acurio have achieved the CE & FDA & ANVISA certificates for our products , and our factory obtained ISO-13485 system certificates. Our products have been sold to more than 110 countries and regions all over the world including Europe, South America, North America and Asia. Acurio provides OEM and ODM services with competitive prices and reliable quality.
Acurio love to learn and are thrilled whenever customers share their experiences with us. Acurio also has the confidence in providing full support to our customers to gain more share in their market. 
Quality Control 
In accordance with the requirements of GMP and ISO13485, a quality management system has been established to manage the company's daily operations, product R&D, manufacturing, quality control, etc. Through the management and control of supplier evaluation, incoming material inspection, process inspection, finished product inspection and other processes, Ensure products are produced for intended use and regulatory requirements, improve the company's overall performance and capabilities, and create value for customers and other relevant parties.
At present, Acurio has passed the IS013485 international medical device quality management system audit and Brazil INMETRO. Our products have passed CE, FDA, NMPA, ANVISA (Brazil) and other certifications, and has earned a reputation for reliable quality and services.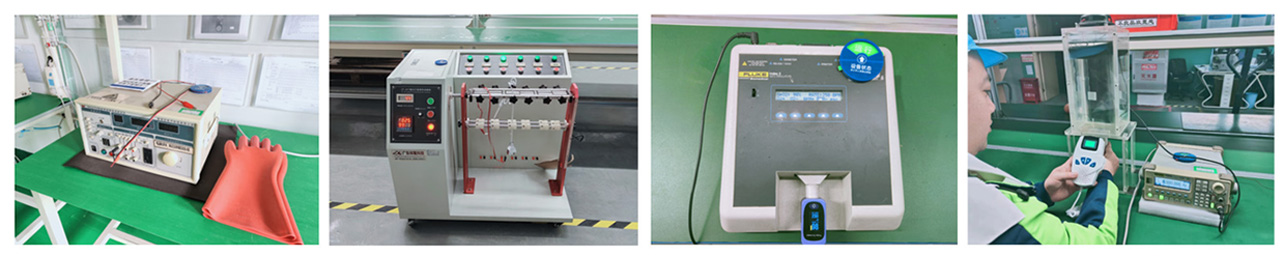
Milestones
2013
Acurio was founded, granted a medical device production licence in China set up R&D projects for multi-parameter physiological detector
2014
Set up R&D projects for Infrared thermometer and compressor nebulizer passed ISO13485 (quality management system) audit for pulse oximeter and blood pressure Passed CFDA audit for blood pressure
2015
Obtained CE certificates for Blood oximeter and blood pressure obtained China Class II medical device business license set up R&D project for fetal doppler
2016
Passed INMETRO (Brazil) audit, registed in ANVISA Brazil passed ISO13485 audit for Compressor Nebulizer Passed CFDA audit for Infrared thermometer
2017
Obtained CE certificates for Fetal doppler and Compressor nebulizer, Obtained FDA certificate for fingertip pulse oximeter
2018
Passed CFDA audit for compressor nebulizer and fetal doppler Acurio was rated as a national high-tech enterprise
2019-2022
During the covid-19 epidemic, Acurio supplies oximeters to many countries in Europe and South America
2023
launched a New product of DC Portable Compressor Nebulizer